Graphene Studies
-
Michael V
- Posts: 479
- Joined: Thu Feb 09, 2012 4:36 pm
- Location: Wales
Re: Graphene Studies
webolife,
Absolutely all photonic "reflection" is a "re-emission" process. At the sub-atomic and aethereal level, there are no surfaces available to facilitate reflection in the manner that we are used to. The incoming aethereal photon "stimulates" and aligns electrons causing what we identify as reflection. Re-emission is not 100% inevitable, that is, reflection is not 100% efficient, some loss is unavoidable.
Michael
Absolutely all photonic "reflection" is a "re-emission" process. At the sub-atomic and aethereal level, there are no surfaces available to facilitate reflection in the manner that we are used to. The incoming aethereal photon "stimulates" and aligns electrons causing what we identify as reflection. Re-emission is not 100% inevitable, that is, reflection is not 100% efficient, some loss is unavoidable.
Michael
- webolife
- Posts: 2539
- Joined: Mon Mar 17, 2008 2:01 pm
- Location: Seattle
Re: Graphene Studies
We significantly agree here, except for our understandings of the term "photon".
I would add that there is no absolutely noncompressable surface. When you press on it, it has to give, diffuse or deform in some manner, and entropy will win over. Now I'm not sure how that would apply to your teeny quantums, but I don't want to go off thread with more debate on that subject.
I would add that there is no absolutely noncompressable surface. When you press on it, it has to give, diffuse or deform in some manner, and entropy will win over. Now I'm not sure how that would apply to your teeny quantums, but I don't want to go off thread with more debate on that subject.
Truth extends beyond the border of self-limiting science. Free discourse among opposing viewpoints draws the open-minded away from the darkness of inevitable bias and nearer to the light of universal reality.
-
seasmith
- Posts: 2815
- Joined: Thu Mar 27, 2008 6:59 pm
Re: Graphene Studies
"Graphene's behavior depends on where it sits"
http://www.nanowerk.com/news2/newsid=26304.php?
They were "surprised" ? Did they really believe that it was a two-dimensional matrerial ??... new experiments conducted at MIT show that a one-atom-thick material called graphene, a form of pure carbon whose atoms are joined in a chicken-wire-like lattice, behaves quite differently depending on the nature of material it’s wrapped around. When sheets of graphene are placed on substrates made of different materials, fundamental properties — such as how the graphene conducts electricity and how it interacts chemically with other materials — can be drastically different, depending on the nature of the underlying material.
“We were quite surprised” to discover this altered behavior, says Michael Strano, the Charles and Hilda Roddey Professor of Chemical Engineering at MIT, who is the senior author of a paper published this week in the journal Nature Chemistry ("Understanding and controlling the substrate effect on graphene electron-transfer chemistry via reactivity imprint lithography").
Think cymatics.“We expected it to behave like graphite” — a well-known form of carbon, used to make the lead in pencils, whose structure is essentially multiple layers of graphene piled on top of each other.
But its behavior turned out to be quite different. “Graphene is very strange,” Strano says. Because of its extreme thinness, in practice graphene is almost always placed on top of some other material for support. When that material underneath is silicon dioxide, a standard material used in electronics, the graphene can readily become “functionalized” when exposed to certain chemicals. But when graphene sits on boron nitride, it hardly reacts at all to the same chemicals.
“It’s very counterintuitive,” Strano says. “You can turn off and turn on graphene’s ability to form chemical bonds, based on what’s underneath.”
The reason, it turns out, is that the material is so thin that the way it reacts is strongly affected by the electrical fields of atoms in the material beneath it.[ No kidding...] This means that it is possible to create devices with a micropatterned substrate — made up of some silicon dioxide regions and some coated with boron nitride — covered with a layer of graphene whose chemical behavior will then vary according to the hidden patterning.
Now there's an idea ...Strano says. For example, the one-atom-thick material, when bonded to copper, completely eliminates that metal’s tendency to oxidize (which produces the characteristic blue-green surface of copper roofs). “It can completely turn off the corrosion,” he says, “almost like magic … with just the whisper of a coating.”
To explain why graphene behaves the way it does, “we came up with a new electron-transfer theory”
http://www.nanowerk.com/news2/newsid=26304.php?
-
seasmith
- Posts: 2815
- Joined: Thu Mar 27, 2008 6:59 pm
Re: Graphene Studies
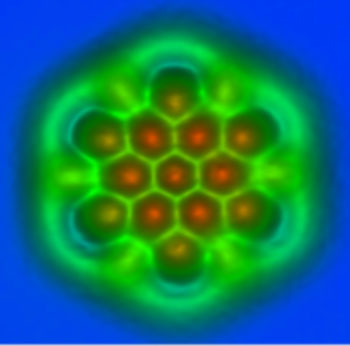
Motions of charge quanta transduced over (a) duration are helicoidal in time-stop schematics, but are more intricately fractal in active progression.
note: [The OP continues this frayed thread solely due the virtual 2D screen shot that graphene provides for 4D electrical phenomena].
... said IBM scientist Leo Gross. “The second contrast mechanism really came as a surprise: Bonds appeared with different lengths in AFM measurements. With the help of ab initio calculations we found that the tilting of the carbon monoxide molecule at the tip apex is the cause of this contrast.”
.IBM Research scientists imaged the bond order and length of individual carboncarbon bonds in C60, also known as a buckyball for its football shape and two planar polycyclic aromatic hydrocarbons (PAHs), which resemble small flakes of graphene
As in their earlier research ("The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy") the IBM scientists used an atomic force microscope (AFM) with a tip that is terminated with a single carbon monoxide (CO) molecule. This tip oscillates with a tiny amplitude above the sample to measure the forces between the tip and the sample, such as a molecule, to create an image. The CO termination of the tip acts as a powerful magnifying glass to reveal the atomic structure of the molecule, including its bonds. This made it possible to distinguish individual bonds that differ only by 3 picometers or 3 × 10-12 meters, which is about one-hundredth of an atom’s diameter.
errghh??Comparison with theory shows that Pauli repulsion is the source of the atomic resolution, whereas van der Waals and electrostatic forces only add a diffuse attractive background.
http://www.sciencemag.org/content/325/5 ... /Molecular Resonance
http://www.nanowerk.com/news2/newsid=26 ... oo%21+Mail
http://en.wikipedia.org/wiki/Resonance
Perhaps this tilt is commensurate with MJV'sThereby they calculated the tilting of the CO molecule at the tip apex that occurs during imaging.
http://www.thunderbolts.info/wp/forum/phpB ... 445#p70445a process of mediation by an aethereal field,
a characterization with which i would not strongly disagree.
MJV,
While i'm fractionally impressed that you've pretty much adopted an earlier expressed translation of frequency-harmonic "attraction",
there seems to remain some dis-connection between thine connection of mechanical and harmonical.
s
-
seasmith
- Posts: 2815
- Joined: Thu Mar 27, 2008 6:59 pm
Re: Graphene Studies
Room Temperature Superconductivity Found in Graphite Grains
http://www.technologyreview.com/view/42 ... 2012-09-17So that's a surface effect which involves only a tiny fraction of the total mass of carbon in the powder--just 0.0001 per cent of the mass, according to Esquinazi and co.
-
seasmith
- Posts: 2815
- Joined: Thu Mar 27, 2008 6:59 pm
Re: Graphene Studies
~
The SI Abyss
http://www.nanowerk.com/news2/newsid=30 ... oo%21+Mail
The SI Abyss
[like, what and why is a "quantum", a quantum]Graphene joins the race to redefine the ampere
A new joint innovation by the National Physical Laboratory (NPL) and the University of Cambridge could pave the way for redefining the ampere in terms of fundamental constants of physics. The world's first graphene single-electron pump (SEP), described in a paper today in Nature Nanotechnology ("Gigahertz quantized charge pumping in graphene quantum dots"), provides the speed of electron flow needed to create a new standard for electrical current based on electron charge.
The international system of units (SI) comprises seven base units (the metre, kilogram, second, Kelvin, ampere, mole and candela).
[no unit for magnetism ??]
Ideally these should be stable over time and universally reproducible. This requires definitions based on fundamental constants of nature which are the same wherever you measure them.
The present definition of the Ampere, however, is vulnerable to drift and instability. This is not sufficient to meet the accuracy needs of present and certainly future electrical measurement. The highest global measurement authority, the Conférence Générale des Poids et Mesures, has proposed that the ampere be re-defined in terms of the electron charge.
...
A good SEP pumps precisely one electron at a time to ensure accuracy, and pumps them quickly to generate a sufficiently large current. Up to now the development of a practical electron pump has been a two-horse race. Tuneable barrier pumps use traditional semiconductors and have the advantage of speed, while the hybrid turnstile utilises superconductivity and has the advantage that many can be put in parallel. Traditional metallic pumps, thought to be not worth pursuing, have been given a new lease of life by fabricating them out of the world's most famous super-material - graphene.
Previous metallic SEPs made of aluminium are very accurate, but pump electrons too slowly for making a practical current standard. Graphene's unique semimetallic two-dimensional structure has just the right properties to let electrons on and off the quantum dot very quickly, creating a fast enough electron flow - at near gigahertz frequency - to create a current standard. The Achillies heel of metallic pumps, slow pumping speed, has thus been overcome by exploiting the unique properties of graphene.
...
The realisation of the ampere is currently derived indirectly from resistance or voltage, which can be realised separately using the quantum Hall effect and the Josephson Effect. A fundamental definition of the ampere would allow a direct realisation that National Measurement Institutes around the world could adopt. This would shorten the chain for calibrating current-measuring equipment, saving time and money for industries billing for electricity and using ionising radiation for cancer treatment.
...Also, any redefinition will have to wait until the Kilogram has been redefined. This definition, due to be decided soon, will fix the value of electronic charge, on which any electron-based definition of the ampere will depend.Current, voltage and resistance are directly correlated. Because we measure resistance and voltage based on fundamental constants – electron charge and Planck's constant -
[constant ??]
being able to measure current would also allow us to confirm the universality of these constants on which many precise measurements rely.
,,, and for answering fundamental questions in quantum mechanics.
http://www.nanowerk.com/news2/newsid=30 ... oo%21+Mail
-
seasmith
- Posts: 2815
- Joined: Thu Mar 27, 2008 6:59 pm
Re: Graphene Studies
~
Magnetic Deflagration
Solar Flares, CME's anyone ?
http://prl.aps.org/abstract/PRL/v110/i20/e207203
http://www.nanowerk.com/news2/newsid=30466.php?
Magnetic Deflagration
In this case, they employed small single crystals of a molecular magnet— each magnetic molecule being just one billionth of a meter—that could be magnetized, much like the needle of a compass.
The researchers provided a pulse of heat as the spark, causing molecular spins near the heaters to flip in a magnetic field, a process that released energy and transmitted it to nearby material.
“When the molecules’ spins are aligned opposite the applied field direction, they possess a high level of energy,” explained Andrew Kent, a professor in NYU’s Department of Physics and the study’s senior researcher. “And then when the spins ‘flip,’ energy is released and dispersed into surrounding magnetic material that can cause a runaway reaction.”
Solar Flares, CME's anyone ?
http://prl.aps.org/abstract/PRL/v110/i20/e207203
http://www.nanowerk.com/news2/newsid=30466.php?
-
seasmith
- Posts: 2815
- Joined: Thu Mar 27, 2008 6:59 pm
Re: Graphene Studies
¶
Graphene's love affair with water
http://www.nanowerk.com/nanotechnology_ ... z2tLkUT1jk
There seems to be instruction on flow of elemental charge here, maybe having to do with atomically aligned conducive surfaces (inner walls of "capillaries", by definition entail an inner surface alignment), but a coherent explanation currently eludes me.
http://www.sciencemag.org/content/343/6172/752
Graphene's love affair with water
Graphene is hydrophobic – it repels water – but narrow capillaries made from graphene vigorously suck in water allowing its rapid permeation, if the water layer is only one atom thick – that is, as thin as graphene itself.
One-atom-wide graphene capillaries can now be made easily and cheaply by piling layers of graphene oxide – a derivative of graphene – on top of each other. The resulting multilayer stacks (laminates) have a structure similar to nacre (mother of pearl), which makes them also mechanically strong.
… [and] impermeable to all gases and vapours, except for water. This means that even helium, the hardest gas to block off, cannot pass through the membranes whereas water vapour went through with no resistance.
...
Small salts with a size of less than nine Angstroms can flow along but larger ions or molecules are blocked. Ten Angstroms is equivalent to a billionth of a metro.
The graphene filters have an astonishingly accurate mesh that allows them to distinguish between atomic species that are only a few percent different in size.
On top of this ultraprecise separation, it is also ultrafast. Those ions that can go through do so with such a speed as if the graphene membranes were an ordinary coffee filter.
http://www.nanowerk.com/nanotechnology_ ... z2tLkUT1jk
There seems to be instruction on flow of elemental charge here, maybe having to do with atomically aligned conducive surfaces (inner walls of "capillaries", by definition entail an inner surface alignment), but a coherent explanation currently eludes me.
What is the nano-nature of this ionic pressure ?Graphene-based materials can have well-defined nanometer pores and can exhibit low frictional water flow inside them, making their properties of interest for filtration and separation. We investigate permeation through micrometer-thick laminates prepared by means of vacuum filtration of graphene oxide suspensions. The laminates are vacuum-tight in the dry state but, if immersed in water, act as molecular sieves, blocking all solutes with hydrated radii larger than 4.5 angstroms. Smaller ions permeate through the membranes at rates thousands of times faster than what is expected for simple diffusion. We believe that this behavior is caused by a network of nanocapillaries that open up in the hydrated state and accept only species that fit in. The anomalously fast permeation is attributed to a capillary-like high pressure acting on ions inside graphene capillaries.
http://www.sciencemag.org/content/343/6172/752
- webolife
- Posts: 2539
- Joined: Mon Mar 17, 2008 2:01 pm
- Location: Seattle
Re: Graphene Studies
Does anyone know if Dr. Gerry Pollock has investigated graphene as a hydrophillic structured water gel-producing surface? I wonder if this might help explain some of the ionic attributes of graphene?
Truth extends beyond the border of self-limiting science. Free discourse among opposing viewpoints draws the open-minded away from the darkness of inevitable bias and nearer to the light of universal reality.
-
seasmith
- Posts: 2815
- Joined: Thu Mar 27, 2008 6:59 pm
Re: Graphene Studies
`
Waving Potential
As opined before, all material interfaces are electric double-layers. It seems the atom thick graphene honeycomb structure may act as a mesh/net, collecting nascent charge from the double-layer ? and conducting it to the electroscope.
http://www.nature.com/ncomms/2014/14050 ... s4582.html
Waving Potential
experiments have demonstrated that flowing water over carbon nanotubes can generate electric voltages. However, the reported flow-induced voltages are in wide discrepancy and the proposed mechanisms remain conflictive. Here we find that moving a liquid–gas boundary along a piece of graphene can induce a waving potential of up to 0.1 V. The potential is proportional to the moving velocity and the graphene length inserted into ionic solutions, but sharply decreases with increasing graphene layers and vanishes in other materials. This waving potential arises from charge transfer in graphene driven by a moving boundary of an electric double layer between graphene and ionic solutions.
The results reveal a unique electrokinetic phenomenon and open prospects for functional sensors, such as tsunami monitors.
This moving gas-liquid boundary [electric double-layer] induces charge transfer in graphene,
As opined before, all material interfaces are electric double-layers. It seems the atom thick graphene honeycomb structure may act as a mesh/net, collecting nascent charge from the double-layer ? and conducting it to the electroscope.
http://www.nature.com/ncomms/2014/14050 ... s4582.html
Who is online
Users browsing this forum: No registered users and 13 guests